Cellular behavior is governed by a constant flow of signals originating from the extracellular environment and internal cellular states. These signals must be accurately perceived, integrated, and translated into appropriate biological responses. Central to this process are signal transduction pathways and transcriptional regulators, which together determine how cells adapt, differentiate, survive, or respond to stress.
Among the many signaling pathways described in molecular biology, the p38 mitogen-activated protein kinase (MAPK) pathway has emerged as a critical mediator of stress responses and differentiation processes. In parallel, the transcription factor MEF2C (Myocyte Enhancer Factor 2C) plays a pivotal role in regulating gene expression programs across multiple tissues.
The functional relationship between p38 signaling and MEF2C activity represents a compelling example of how extracellular cues are converted into precise transcriptional outcomes. This article explores the molecular basis and biological significance of the p38–MEF2C axis, highlighting its role in cellular regulation and biological complexity
p38 MAPK: a stress-activated signaling hub
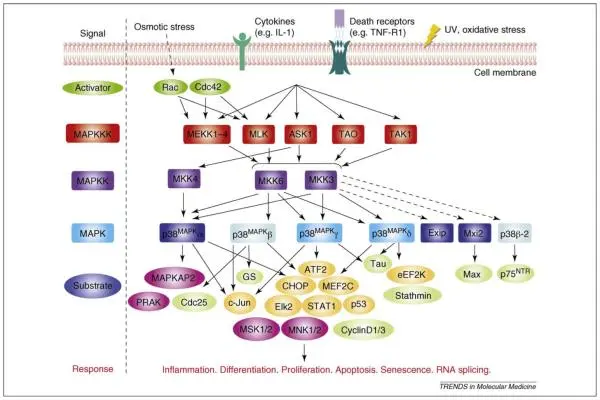
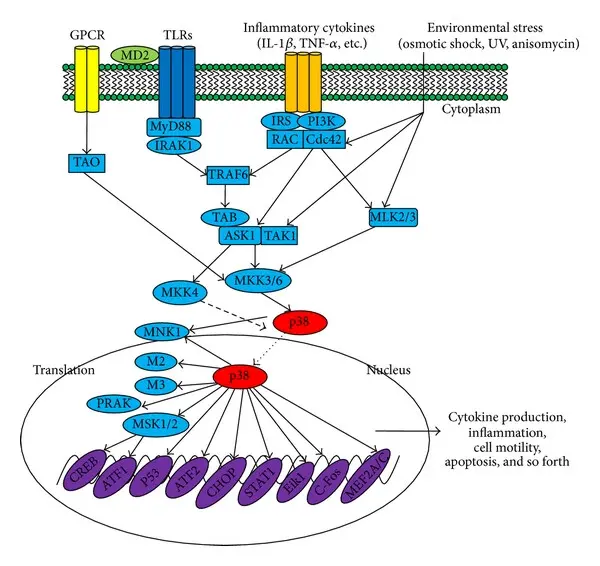
The p38 MAPK pathway belongs to the broader family of MAP kinases, which also includes ERK and JNK pathways. Unlike ERK, which is primarily associated with mitogenic signals, p38 is predominantly activated in response to cellular stress.
Activation and regulation of p38
p38 MAPK is activated through phosphorylation by upstream kinases in response to various stimuli, including:
inflammatory cytokines
oxidative stress
osmotic shock
UV irradiation
metabolic stress
Once activated, p38 phosphorylates a diverse set of substrates, including kinases, transcription factors, and regulatory proteins. This versatility positions p38 as a central signaling hub, capable of influencing multiple cellular processes depending on context.
Context-dependent signaling
Importantly, p38 activation does not lead to a single, uniform cellular response. The biological outcome depends on several factors:
Cell type
Intensity and duration of the signal
Interaction with parallel signaling pathways
Availability of downstream effectors
This context dependency is a defining feature of p38 signaling and underscores the complexity of its biological roles.
p38 MAPK: a stress-activated signaling hub
The p38 MAPK pathway belongs to the broader family of MAP kinases, which also includes ERK and JNK pathways. Unlike ERK, which is primarily associated with mitogenic signals, p38 is predominantly activated in response to cellular stress.
Activation and regulation of p38
p38 MAPK is activated through phosphorylation by upstream kinases in response to various stimuli, including:
inflammatory cytokines
oxidative stress
osmotic shock
UV irradiation
metabolic stress
Once activated, p38 phosphorylates a diverse set of substrates, including kinases, transcription factors, and regulatory proteins. This versatility positions p38 as a central signaling hub, capable of influencing multiple cellular processes depending on context.
Context-dependent signaling
Importantly, p38 activation does not lead to a single, uniform cellular response. The biological outcome depends on several factors:
cell type
intensity and duration of the signal
interaction with parallel signaling pathways
availability of downstream effectors
This context dependency is a defining feature of p38 signaling and underscores the complexity of its biological roles.
MEF2C: a transcriptional integrator of cellular signals
MEF2C is a member of the MEF2 family of transcription factors, characterized by their conserved DNA-binding domain and their ability to regulate genes involved in differentiation, development, and cellular function.
Biological roles of MEF2C
MEF2C plays essential roles in multiple biological systems, including:
muscle development and differentiation
neuronal maturation and synaptic function
hematopoiesis and immune cell differentiation
cardiovascular development
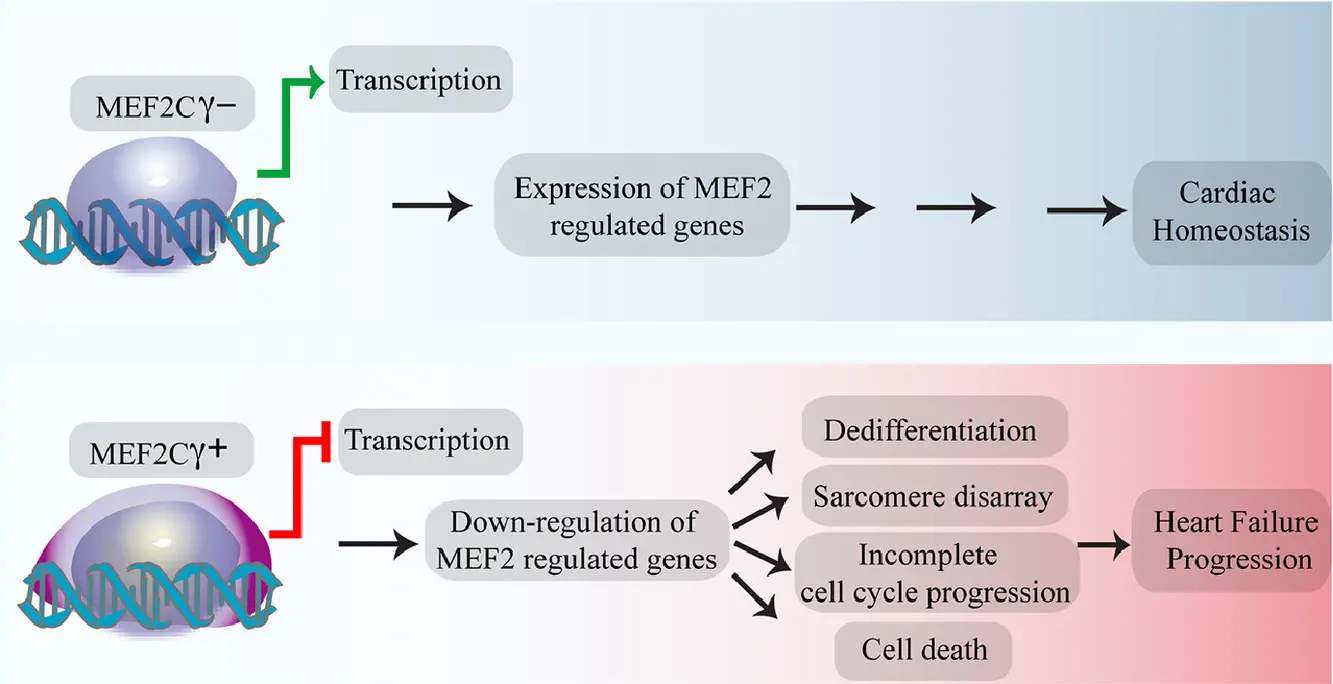
Across these contexts, MEF2C acts as a master regulator, controlling gene expression programs required for cell identity and function.
Regulation of MEF2C activity
The activity of MEF2C is tightly regulated at multiple levels:
transcriptional control of MEF2C expression
post-translational modifications
interactions with co-activators and co-repressors
modulation by upstream signaling pathways
This multilayered regulation allows MEF2C to respond dynamically to cellular signals, making it an ideal node for signal-dependent transcriptional control.
Linking signaling to transcription: the p38–MEF2C axis
The functional connection between p38 signaling and MEF2C activity provides a clear illustration of how extracellular stimuli influence gene expression..
Molecular mechanisms of interaction
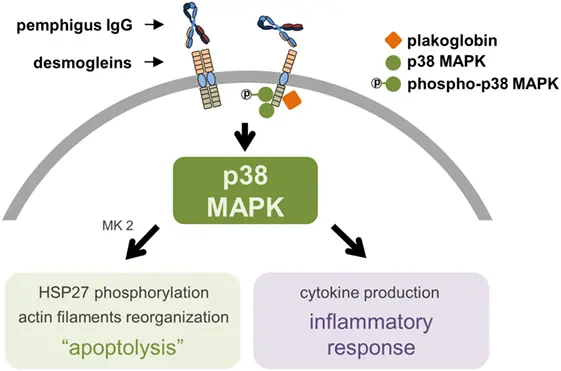
Activated p38 can modulate MEF2C activity through several mechanisms, most notably via phosphorylation events that enhance MEF2C’s transcriptional potential. These modifications can:
increase MEF2C’s ability to recruit co-activators
alter its interaction with chromatin-associated factors
fine-tune the expression of target genes
Rather than acting as a simple on/off switch, p38-dependent regulation of MEF2C introduces gradual and context-sensitive modulation of transcriptional programs.
Targeting of p38 Mitogen-Activated Protein Kinases to MEF2 Transcription Factors.pdf
Functional consequences
Through MEF2C, p38 signaling influences a wide range of biological outcomes, such as:
initiation or maintenance of differentiation programs
adaptation to environmental stress
regulation of survival and apoptosis-related genes
coordination of developmental and stress-responsive pathways
This coupling allows cells to align transcriptional decisions with their physiological state.
Biological significance across systems
The relevance of the p38–MEF2C axis extends across multiple biological contexts.
Differentiation and development
During differentiation, cells must integrate signals that promote lineage commitment while suppressing alternative fates. The p38–MEF2C axis contributes to this process by coordinating signaling inputs with transcriptional outputs, ensuring coherent gene expression programs.
Stress adaptation
Cells exposed to stress must rapidly adjust their transcriptional landscape. By linking stress-activated p38 signaling to MEF2C-dependent gene regulation, cells can mount adaptive responses that preserve function and viability.
Issue-specific roles
Tissue-specific roles
Despite the conserved nature of p38 and MEF2C, their interaction leads to tissue-specific outcomes. This specificity arises from differences in:
chromatin landscape
co-regulatory protein availability
signaling network architecture
Complexity and limitations of reductionist interpretations
While the p38–MEF2C axis provides a useful framework, it should not be viewed as a linear or isolated pathway. Biological systems operate through networks of interactions, where multiple signaling cascades converge on shared transcriptional regulators.
Focusing exclusively on single pathways risks oversimplifying:
cross-talk with other MAPK pathways
feedback mechanisms
compensatory regulatory processes
A comprehensive understanding requires integrating multiple layers of regulation and acknowledging system-level complexity.
From molecular mechanisms to biological insight
The study of the p38–MEF2C axis exemplifies a broader principle in modern biology: mechanistic understanding emerges from the integration of signaling, transcription, and context.
Rather than treating signaling pathways and transcription factors as independent entities, this perspective emphasizes their functional coupling and biological interdependence.
Such an approach enables:
more accurate interpretation of experimental data
better alignment between molecular observations and biological outcomes
generation of robust, biologically meaningful conclusions
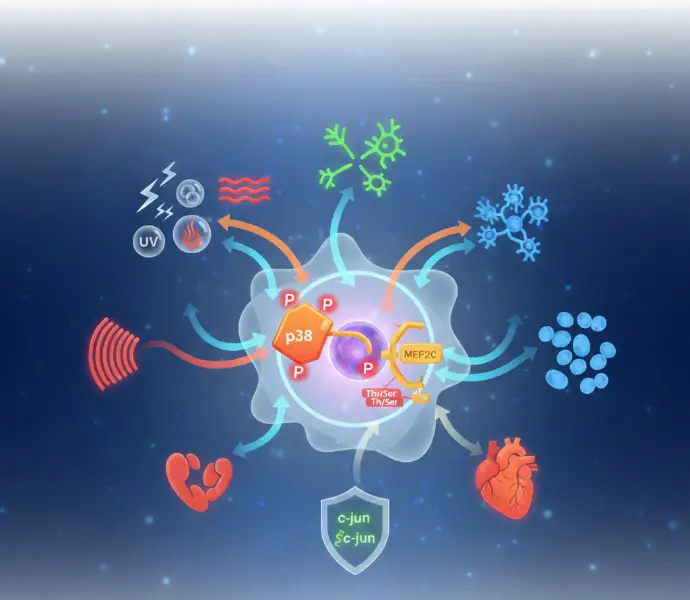
Conclusion
The p38–MEF2C axis represents a compelling example of how cells translate external and internal cues into structured transcriptional responses. By linking stress-activated signaling to context-dependent gene regulation, this axis plays a crucial role in differentiation, adaptation, and cellular decision-making.
Understanding such regulatory relationships requires moving beyond linear models toward integrated, context-aware interpretations of biological systems. In doing so, researchers gain deeper insight into the principles governing cellular behavior and biological complexity.